Start treatment with CRYSVITA® as early as possible after diagnosis of XLH in suitable patients
Table of Contents
As of 10 September 2021, CRYSVITA® has been approved by Therapeutic Goods Administration (TGA) for the treatment of X-linked hypophosphataemia (XLH) in adults, adolescents and children 1 year of age or older.1 Full prescibing information can be found here
As of 1 November 2022, CRYSVITA® has been listed on the Pharmaceutical Benefits Scheme. PBS Information: This product is listed on the PBS as a Section 100 item. Refer to the PBS Schedule for full authority information.
Dosing
CRYSVITA® dosing is every 2 weeks in paediatric patients (1 to less than 18 years of age)1
As of 3 May 2021, CRYSVITA has been approved by Health Science Authority (HSA) Singapore. To decide if this is a suitable option for any of your patients, please click here for more information.
Product is approved in EU/US/Japan and selected markets in Asia Pacific. Local approved prescribing information may differ.
Please refer to local approval status and prescribing information.
Dosing in paediatric patients (children aged 1-11 years and adolescents aged 12-17 years of age) is every 2 weeks.
The recommended starting dose regimen is 0.8 mg/kg of body weight, rounded to the nearest 10 mg, every 2 weeks. The minimum starting dose is 10 mg up to a maximum dose of 90 mg. All doses should be rounded to the nearest 10 mg.
After initiating CRYSVITA®, measure fasting serum phosphate:
- every 4 weeks for the first 3 months of treatment, and
- thereafter as appropriate.
If serum phosphate is within the reference range for age, continue with the same dose. Follow dose adjustment schedule below to maintain serum phosphate within the reference range for age.
Fasting serum phosphate concentration should be below the reference range for age prior to initiation of treatment.
Discontinue oral phosphate and active vitamin D analogues (e.g. calcitriol, paricalcitol, doxercalciferol, calcifediol) 1 week prior to initiation of treatment . Vitamin D replacement or supplementation with inactive forms of vitamin D may be continued as per local guidelines.
CRYSVITA® is dosed every 4 weeks in adult patients (18 years of age and older)1
Dosing in adults is every 4 weeks.
The recommended dose regimen in adults is 1 mg/kg of body weight, rounded to the nearest 10 mg up to a maximum dose of 90 mg, administered every 4 weeks.
After initiation of treatment with CRYSVITA®, fasting serum phosphate should be measured:
- on a monthly basis, measured 2 weeks post-dose, for the first 3 months of treatment, and
- thereafter as appropriate.
If serum phosphate is within the normal range, continue with the same dose.
Discontinue oral phosphate and active vitamin D analogs 1 week prior to initiation of treatment. Vitamin D replacement or supplementation with inactive forms of vitamin D may be continued as per local guidelines. Fasting serum phosphate concentration should be below the reference range for age prior to initiation of treatment.
Calculation for starting dose
Sample Starting Dose Calculation – Paediatric (1 to <18 years old)
Patient weight (kg) x Recommended starting dose (0.8mg/kg)
Example: 23kg x 0.8mg/kg = 18.4 mg (Round to nearest 10 mg)
Starting dose CRYSVITA® = 20 mg
Sample Starting Dose Calculation – Adult patient (≥18 years of age)
Patient weight (kg) x Recommended starting dose (1mg/kg)
Example: 77kg x 1mg/kg = 77 mg (Round to nearest 10 mg)
Starting dose CRYSVITA® = 80 mg
Paediatrics Dose Adjustments1
Dose Increase (children aged 1-11 years and adolescents aged 12-17 years of age)1 :
If serum phosphate is below the reference range for age, the dose may be increased stepwise up to a maximum of 2.0 mg/kg, rounded to the nearest 10 mg, administered every 2 weeks (maximum dose of 90 mg) according to the Paediatric Dose Schedule for Stepwise Dose Increase dosing schedule shown below.
Table 1. PAEDIATRIC DOSE SCHEDULE
Paediatric Dose Schedule for Stepwise Dose Increase
| Body Weight (kg) | Starting Dose (mg) | First Dose Increase to (mg) | Second Dose Increase to (mg) |
|---|---|---|---|
| 10-14 | 10 | 15* | 20 |
| 15-18 | 10 | 20 | 30 |
| 19-31 | 20 | 30 | 40 |
| 32-43 | 30 | 40 | 60 |
| 44-56 | 40 | 60 | 80 |
| 57-68 | 50 | 70 | 90 |
| 69-80 | 60 | 90 | 90 |
| 81-93 | 70 | 90 | 90 |
| 94-105 | 80 | 90 | 90 |
|
| 90 | 90 | 90 |
*This does increase is an exception to rounding to the nearest 10mg
Dose decrease (pediatric patients 1 to less than 18 years of age)1
Dose decrease (children aged 1-11 years and adolescents aged 12-17 years of age)1 :
If serum phosphate is above the reference range for age, withhold the next dose and reassess the serum phosphate level within 4 weeks. The patient must have serum phosphate below the reference range for age to restart CRYSVITA® . Once serum phosphate is below the reference range for age, treatment may be restarted according to the Paediatric Dose Schedule for Re-Initiation of Therapy dosing schedule shown below.
Reassess serum phosphate level 4 weeks after dose adjustment. If serum phosphate is below the reference range for age 4 weeks after dose adjustment, the dose can be restarted at 0.8 mg/kg every 2 weeks.
Table 2. PAEDIATRIC DOSE SCHEDULE
Paediatric Dose Schedule for Re-Initiation of Therapy
| Previous Dose (mg) | Re-initiation Dose (mg) |
|---|---|
| 10 | 5 |
| 15 | 10 |
| 20 | 10 |
| 30 | 10 |
| 40 | 20 |
| 50 | 20 |
| 60 | 30 |
| 70 | 30 |
| 80 | 40 |
| 90 | 40 |
Dose decrease (adults)1
Dose Decrease (adults 18 years of age and older)1 :
If serum phosphate is above the normal range, withhold the next dose and reassess the serum phosphate level within 4 weeks. The patient must have serum phosphate below the normal range to be able to restart CRYSVITA® . Once serum phosphate is below the normal range, treatment may be restarted at half the initial starting dose up to a maximum dose of 40 mg every 4 weeks according to the Adult Dose Schedule for Re-Initiation of Therapy dosing schedule shown below.
Reassess fasting serum phosphate level 2 weeks after dose adjustment.
Do not adjust burosumab more frequently than every 4 weeks.
Table 3. ADULT DOSE SCHEDULE FOR RE-INITIATION OF THERAPY
| Previous Dose (mg) | Re-initiation Dose (mg) |
|---|---|
| 40 | 20 |
| 50 | 20 |
| 60 | 30 |
| 70 | 30 |
|
| 40 |
Download the CRYSVITA® Dosing Guide for additional information on initiating, dosing, and administering CRYSVITA®.
Missed or late dosing1
If a patient misses a dose, resume CRYSVITA® as soon as possible at prescribed dose. To avoid missed doses, treatments may be administered 3 days either side of the scheduled treatment date.
Administration
CRYSVITA® Administration1
CRYSVITA® should be administered subcutaneously1
Discontinue oral phosphate and active vitamin D analogs 1 week prior to initiation of treatment. Fasting serum phosphate concentration should be below the reference range for age prior to initiation of treatment
For subcutaneous use.
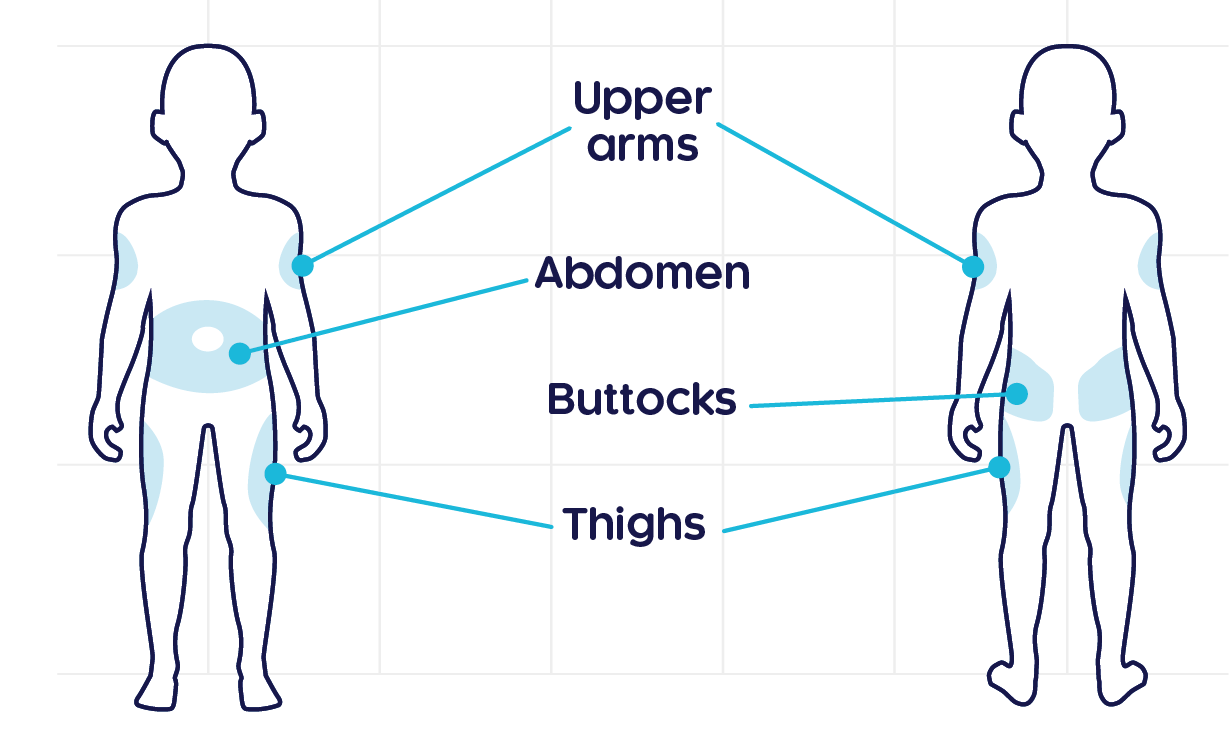
CRYSVITA® should be injected in the upper arm, abdomen, buttock or thigh. Injection sites should be rotated with each injection administered at a different anatomic location than the previous injection. Do not inject into moles, scars, or areas where the skin is tender, bruised, red, hard, or not intact.
The maximum volume of medicinal product per injection site is 1.5 mL. If more than 1.5 mL is required on a given dosing day, the total volume of medicinal product should be split and should be administered at different injection sites. Injection sites should be rotated and carefully monitored for signs of potential reactions.
Although you and your staff may be familiar with the preparation of drug suspensions and the administration of depot injections, it is important that you read all the instructions specifically administering CRYSVITA® . Clinical monitoring of the patient, including monitoring of phosphate levels, must continue as required and as outlined below. A detailed ‘Instructions for Use’ intended for Healthcare Professionals can be found, by clicking here.
Monitoring
Recommended monitoring schedule for CRYSVITA® patients2,3
| Assessment | Frequency |
|---|---|
| Fasting serum phosphate† | Every 4 weeks for the first 3 months of treatment, and thereafter as appropriate |
| Renal ultrasonography | At the start of treatment and every 6 months for the first 12 months of treatment, and annually thereafter |
| Plasma ALP, calcium, PTH and creatinine | Every 6 months (every 3 months for children 1 - 2 years) or as indicated |
| Urine calcium and phosphate‡ | Every 3 months |
†It is recommended that fasting serum phosphate is targeted at the lower end of the normal reference for age.
‡Upper normal range (mol/mol): 2.2 (<1 years), 1.4 (1–3 years), 1.1 (3–5 years), 0.8 (5–7 years) and 0.7 (>7 years).
Contraindications, warnings and precautions1
Contraindications
- Hypersensitivity to the active substance or to any of the excipients
- Concurrent administration with oral phosphate and / or active vitamin D analogues.
- Serum phosphate level within or above the normal range for age at initiation of treatment.
- Severe renal impairment or end stage renal disease
Warnings
Ectopic mineralisation
Ectopic mineralisation, as manifested by nephrocalcinosis, has been observed in patients with XLH treated with oral phosphate and active vitamin D analogues; these medicinal products should be stopped at least 1 week prior to initiating CRYSVITA® treatment. Monitoring for signs and symptoms of nephrocalcinosis, e.g. by renal ultrasonography, is recommended at the start of treatment and every 6 months for the first 12 months of treatment, and annually thereafter.
Monitoring of plasma alkaline phosphatase, calcium, parathyroid hormone (PTH) and creatinine is recommended every 6 months (every 3 months for children 1 – 2 years) or as indicated.
Monitoring of urine calcium and phosphate is suggested every 3 months.
Hyperphosphataemia
Levels of fasting serum phosphate should be monitored due to the risk of hyperphosphataemia. To decrease the risk for ectopic mineralisation, it is recommended that fasting serum phosphate does not exceed the upper limit of the normal reference range for age. Dose interruption and/or dose reduction may be required. Periodic measurement of post prandial serum phosphate is advised.
Serum parathyroid hormone
Increases in serum parathyroid hormone have been observed in some XLH patients during treatment with CRYSVITA® . Periodic measurement of serum parathyroid hormone is advised.
Injection site reactions
Administration may result in local injection site reactions. Administration should be interrupted in any patient experiencing severe injection site reactions.
Hypersensitivity
Discontinue CRYSVITA® if serious hypersensitivity reactions occur.
Special Populations
Pregnancy
There are no available data on burosumab use in pregnant women to inform a drug-associated risk of adverse developmental outcomes. Adverse effects on embryofetal development were observed with CRYSVITA® in animals. CRYSVITA® is not recommended for use during pregnancy. Fetal loss and pre-term births were increased and the gestation period was shortened in pregnant cynomolgus monkeys given CRYSVITA® at 30 mg/kg once every 2 weeks (yielding 64 times the exposure in adults as the maximum recommended clinical dose of 1 mg/kg every 4 weeks). This occurred in conjunction with maternal hyperphosphataemia and placental mineralisation. CRYSVITA® was shown to cross the placenta. Ectopic mineralisation was not observed in fetuses or offspring, and treatment did not produce malformations, or affect growth, development or survival of the offspring.
Lactation
There are no data on the use of burosumab in breastfeeding women. It is unknown whether CRYSVITA® is excreted in human milk, although the presence of the maternal IgG in milk is recognised. A decision must be made whether to discontinue breast feeding or to discontinue / abstain from CRYSVITA® therapy taking into account the benefit of breast feeding for the child, the benefit of the therapy for the woman and the potential risk to the child from exposure to CRYSVITA®.
Paediatric Use
The safety and efficacy of burosumab in children with XLH under 1 year of age has not been established in clinical studies.
Geriatric Use
No conclusion can be made regarding any differences in safety or efficacy between patients over 65 years of age and those under 65 years of age.
Renal Impairment
CRYSVITA® as not been studied in patients with renal impairment. Burosumab must not be given to patients with severe or end stage renal disease.
Storage
CRYSVITA® Storage1
Store CRYSVITA® away from light and in refrigerated conditions1
- CRYSVITA® injection for subcutaneous administration is supplied as a sterile, preservative-free, clear to slightly opalescent and colorless to pale brown-yellow solution. The product is available as one single-dose vial per carton in the following strengths: 10 mg/mL, 20 mg/mL, and 30 mg/mL. Each pack contains 1 mL solution in a clear glass vial with butyl rubber stopper, and aluminium seal.
- Visually inspect CRYSVITA® for particulate matter and discoloration prior to administration. CRYSVITA® is a sterile, preservative-free, clear to slightly opalescent and colorless to pale brown-yellow solution for subcutaneous injection.
- Do not use if the solution is discolored or cloudy or if the solution contains any particles or foreign particulate matter.
*Not all presentations may be available locally
- Store in a refrigerator (2°C to 8°C). Do not freeze.
- Store in original package to protect from light.
- Product is for single use in one patient only. Discard any residue.
- Do not shake the vial before use.
- In Australia, any unused medicine or waste material should be disposed of in accordance with local requirements.
Please refer to the full Prescribing Information for full details before prescribing.
1. Australian Product Information for Crysvita® (burosumab) approved 10 September 2021. https://www.kyowakirin.com/australia/our_medicines/doc/crysvita_product_information_leaflet.pdf. Last updated: Sept 2021 Last accessed: March 2023. 2. Haffner D et al. Nat Rev Nephrol. 2019; 15(7): 435–455. 3. Raja Padidela et al. Endocr Connect. 2020 Oct; 9(10): 1051–1056.






