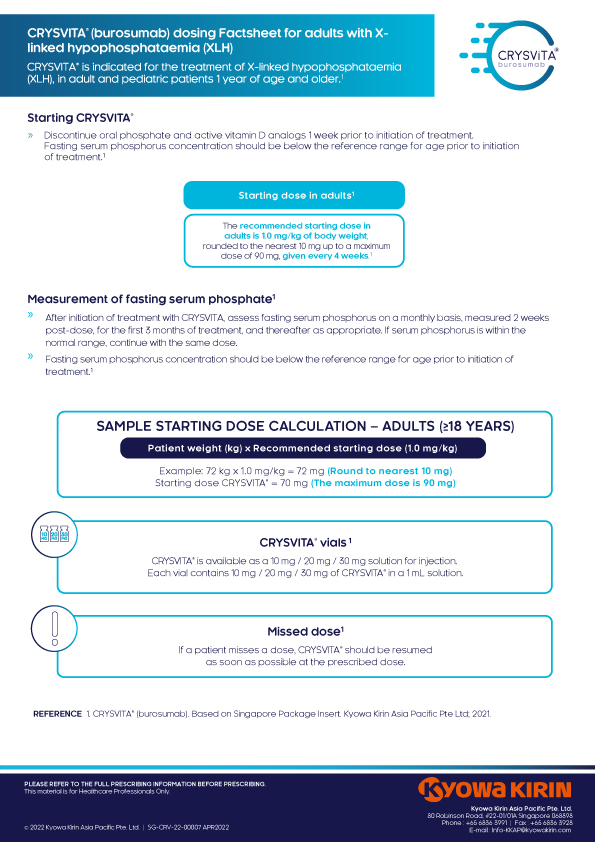
Resources
Downloadable materials
Crysvita® Resources
XLH Resources
Symposium Highlights
Videos
Publications of interest
- Portale AA, et al. Continued beneficial effects of burosumab in adults with X-linked hypophosphatemia: Results from a 24-week treatment continuation period after a 24-week double-blind placebo-controlled period. Calcif Tissue Int. 2019;105:271–84.
PubMed link: https://pubmed.ncbi.nlm.nih.gov/31165191/ - Insogna KL, et al. Burosumab improved histomorphometric measures of osteomalacia in adults with X-linked hypophosphatemia: A phase 3, single-arm, international trial. J Bone Miner Res. 2019;34:2183–91
PubMed link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6916280/ - Haffner D, et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol. 2019;15:435-55.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/31068690 - Imel EA, et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet. 2019;393:2416-27.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/31104833 - Whyte MP, et al. Efficacy and safety of burosumab in children aged 1–4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019;7:189-99.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/30638856 - Beck-Nielsen SS, et al. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J Rare Dis. 2019;14:58.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/30808384 - Insogna KL, et al. A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: Week 24 primary analysis. J Bone Miner Res. 2018;33:1383-93.
PubMed link: https://pubmed.ncbi.nlm.nih.gov/29947083/ - Carpenter TO, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378:1987-98.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/29791829
Key facts
Key Facts about XLH and CRYSVITA®
XLH
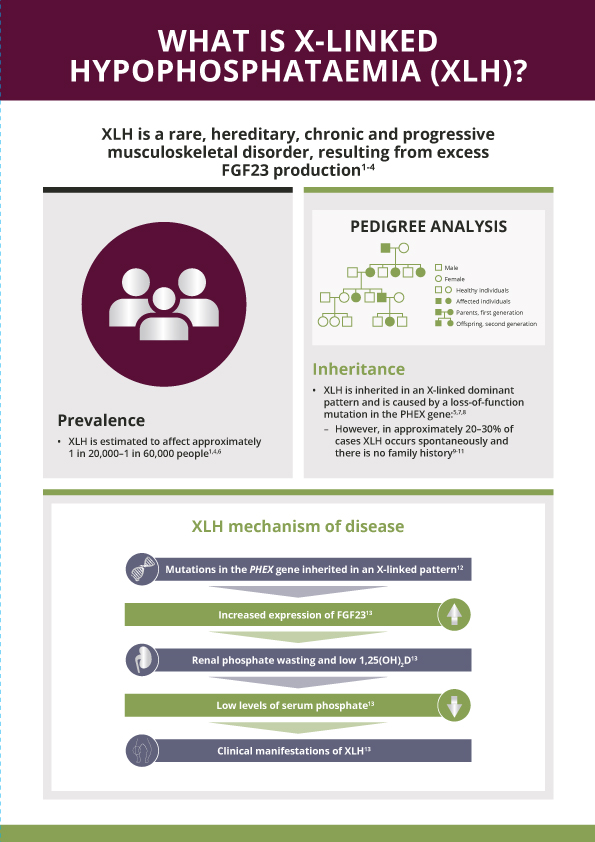
What is XLH?
XLH is a rare, hereditary, progressive and lifelong renal phosphate wasting disorder caused by mutations in the PHEX (phosphate-regulating endopeptidase homolog, X-linked) gene that leads to excess activity of fibroblast growth factor 23 (FGF23)1–4
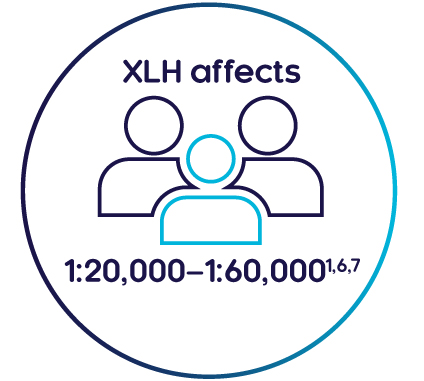
What is the prevalence of XLH?
XLH is a rare disease that affects approximately 1 in 20,000–60,000 people1,5
How is XLH inherited?
XLH is inherited in an X-linked dominant pattern; however, 20–30% of cases arise from spontaneous mutations6,7
What is XLH caused by?
XLH is caused by mutations in the PHEX gene4,5, which is located on the X chromosome
What does it mean for patients with XLH?
Excess FGF23:
- Decreases renal phosphate reabsorption, which increases urinary phosphate excretion8
- Decreases active vitamin D (1,25[OH]2D) production, which reduces intestinal phosphate absorption8
The resulting chronic hypophosphataemia impairs bone mineralisation, leading to a variety of clinical manifestations that can impair patients’ physical function and quality of life9
XLH is not just a bone disease – it is a multisystemic disease that impacts muscles and dentition as well4,10
CRYSVITA®
What is CRYSVITA® ?
CRYSVITA® is a recombinant, fully human monoclonal antibody IgG1 that binds to and inhibits excess FGF23 activity.11 It is the first and only disease-modifying biologic treatment that targets the pathophysiology of XLH.12
How does CRYSVITA® work?
By inhibiting excess FGF23 activity, CRYSVITA® helps restore phosphate homeostasis in people with XLH and improves bone mineralisation mobility and pain.11-14
Who can receive CRYSVITA®?
CRYSVITA® is indicated for the treatment of X-linked hypophosphataemia, in children and adolescents aged 1 to 17 years with radiographic evidence of bone disease, and in adults.11
Why use CRYSVITA®?
The efficacy and safety of CRYSVITA® in children aged 1–12 years, and adults, with XLH have been studied in a global clinical development programme.12–16
A phase 3 clinical study in children with XLH showed that compared with continuing conventional therapy, switching children to CRYSVITA®13:
- Improved phosphate homeostasis
- Significantly improved rickets healing and reduced its severity up to Week 64
- Significantly improved growth and mobility outcomes up to Week 64
- Significantly improved biochemical markers of phosphate regulation and bone health up to Week 64
- In this phase 3 clinical study, CRYSVITA had an acceptable safety profile over 64 weeks in children with XLH13
Phase 3 clinical studies in adults with XLH:
- Phosphate homeostasis, fracture healing, bone mineralisation and remodelling improved, and stiffness were reduced in the CRYSVITA® group compared with the placebo group in a double-blind placebo-controlled study.16
- Phosphate homeostasis improved, and bone quality, mineralisation and remodelling increased in patients treated with CRYSVITA® by Week 48 when compared with that at baseline in a single-arm study.14
- There was more healing of baseline fractures/pseudofractures in patients who continued CRYSVITA® compared with those who received CRYSVITA® after placebo at Week 48 in an open-label study.12
- When placebo-treated patients started CRYSVITA® treatment at Week 24, the healing of fractures/pseudofractures at Week 48 was similar to the healing at Week 24 in those who received CRYSVITA® therapy from the beginning of the study.12
- CRYSVITA® led to sustained improvements in pain, stiffness and physical function and mobility at Week 48 when compared with that at baseline in a double-blind placebo-controlled study.12
- In these phase 3 studies, CRYSVITA® had an acceptable safety profile up to 48 weeks in adults with XLH.12,14
Useful Websites
Publicly Available Websites
XLH information websites for healthcare professionals

1. XLHLink.asia (Healthcare professional website)
https://www.xlhlink.asia/hcp/
This website is developed and funded by Kyowa Kirin Asia Pacific Pte. Ltd.
XLH information websites for healthcare patients

1. XLHLink.asia (Patient website)
https://www.xlhlink.asia/
This website is developed and funded by Kyowa Kirin Asia Pacific Pte. Ltd.
Patient Organisations

1. Malaysian Rare Disorders Society (MRDS)
Formed in 2004, MRDS is a member of RDI and aims to increase awareness of rare disorders through providing information and education.
https://www.mrds.org.my/
1. Beck-Nielsen SS, et al. Eur J Endocrinol. 2009;160:491–7. 2. Endo I, et al. Endocr J. 2015;62:811–6. 3. Carpenter TO, et al. J Bone Miner Res. 2011;26:1381–8. 4. Haffner D, et al. Nat Rev Nephrol. 2019;15:435–55. 5. Rafaelsen S, et al. Eur J Endocrinol. 2016;174:125–36. 6. Rajah J, et al. Eur J Pediatr. 2011;170:1089–96. 7. Raimann A, et al. Wien Med Wochenschr. 2020;170:116–23. 8. Razzaque MS. Nat Rev Endocrinol. 2009;5:611–9. 9. Linglart A, et al. Endocr Connect. 2014;3:R13–30. 10. Beck-Nielsen SS, et al. Orphanet J Rare Dis. 2019;14:58. 11. CRYSVITA® (burosumab) Malaysia Package Insert. September 2022, Version 1. 12. Portale AA, et al. Calcif Tissue Int. 2019;105:271–84. 13. Imel EA, et al. Lancet. 2019;393:2416–27. 14. Insogna KL, et al. J Bone Miner Res. 2019;34:2183–91. 15. Carpenter TO, et al. N Eng J Med. 2018;378:1987–98. 16. Insogna KL, et al. J Bone Miner Res. 2018;33:1383–93