Resources
Downloadable materials
Crysvita® Resources
XLH Resources
Symposium Highlights
Videos
Publications of interest
- Portale AA, et al. Continued beneficial effects of burosumab in adults with X-linked hypophosphatemia: Results from a 24-week treatment continuation period after a 24-week double-blind placebo-controlled period. Calcif Tissue Int. 2019;105:271–84.
PubMed link: https://pubmed.ncbi.nlm.nih.gov/31165191/ - Insogna KL, et al. Burosumab improved histomorphometric measures of osteomalacia in adults with X-linked hypophosphatemia: A phase 3, single-arm, international trial. J Bone Miner Res. 2019;34:2183–91
PubMed link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6916280/ - Haffner D, et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol. 2019;15:435-55.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/31068690 - Imel EA, et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet. 2019;393:2416-27.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/31104833 - Whyte MP, et al. Efficacy and safety of burosumab in children aged 1–4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019;7:189-99.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/30638856 - Beck-Nielsen SS, et al. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J Rare Dis. 2019;14:58.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/30808384 - Insogna KL, et al. A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: Week 24 primary analysis. J Bone Miner Res. 2018;33:1383-93.
PubMed link: https://pubmed.ncbi.nlm.nih.gov/29947083/ - Carpenter TO, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378:1987-98.
PubMed link: https://www.ncbi.nlm.nih.gov/pubmed/29791829
Key facts
Key Facts about XLH and CRYSVITA®
XLH
What is XLH?
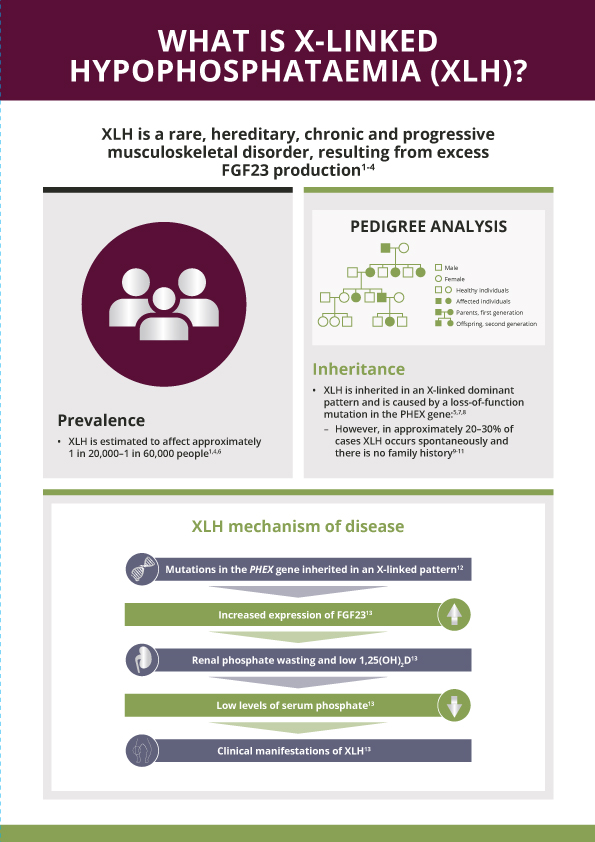
XLH is a rare, hereditary, progressive and lifelong renal phosphate wasting disorder caused by mutations in the PHEX (phosphate-regulating endopeptidase homolog, X-linked) gene that leads to excess activity of fibroblast growth factor 23 (FGF23)1–4
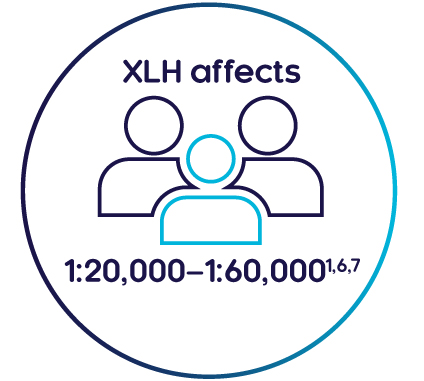
What is the prevalence of XLH?
XLH is a rare disease that affects approximately 1 in 20,000–60,000 people1,5
How is XLH inherited?
XLH is inherited in an X-linked dominant pattern; however, 20–30% of cases arise from spontaneous mutations6,7
What is XLH caused by?
XLH is caused by mutations in the PHEX gene4,5, which is located on the X chromosome
What does it mean for patients with XLH?
Excess FGF23:
- Decreases renal phosphate reabsorption, which increases urinary phosphate excretion8
- Decreases active vitamin D (1,25[OH]2D) production, which reduces intestinal phosphate absorption8
The resulting chronic hypophosphataemia impairs bone mineralisation, leading to a variety of clinical manifestations that can impair patients’ physical function and quality of life9
XLH is not just a bone disease – it is a multisystemic disease that impacts muscles and dentition as well4,10
CRYSVITA®
What is CRYSVITA® ?
CRYSVITA® is a recombinant, fully human monoclonal antibody IgG1 that binds to and inhibits excess FGF23 activity.11 It is the first and only disease-modifying biologic treatment that targets the pathophysiology of XLH.12
How does CRYSVITA® work?
By inhibiting excess FGF23 activity, CRYSVITA® helps restore phosphate homeostasis in people with XLH and improves bone mineralisation mobility and pain.11-14
Who can receive CRYSVITA®?
CRYSVITA® is indicated for the treatment of X-linked hypophosphatemia (XLH) in adult and pediatric patients 1 year of age and older.11
Why use CRYSVITA®?
The efficacy and safety of CRYSVITA® in children aged 1–12 years, and adults, with XLH have been studied in a global clinical development programme.12–16
A phase 3 clinical study in children with XLH showed that compared with continuing conventional therapy, switching children to CRYSVITA®13:
- Improved phosphate homeostasis
- Significantly improved rickets healing and reduced its severity up to Week 64
- Significantly improved growth and mobility outcomes up to Week 64
- Significantly improved biochemical markers of phosphate regulation and bone health up to Week 64
- In this phase 3 clinical study, CRYSVITA had an acceptable safety profile over 64 weeks in children with XLH13
Phase 3 clinical studies in adults with XLH:
- Phosphate homeostasis, fracture healing, bone mineralisation and remodelling improved, and stiffness were reduced in the CRYSVITA® group compared with the placebo group in a double-blind placebo-controlled study.16
- Phosphate homeostasis improved, and bone quality, mineralisation and remodelling increased in patients treated with CRYSVITA® by Week 48 when compared with that at baseline in a single-arm study.14
- There was more healing of baseline fractures/pseudofractures in patients who continued CRYSVITA® compared with those who received CRYSVITA® after placebo at Week 48 in an open-label study.12
- When placebo-treated patients started CRYSVITA® treatment at Week 24, the healing of fractures/pseudofractures at Week 48 was similar to the healing at Week 24 in those who received CRYSVITA® therapy from the beginning of the study.12
- CRYSVITA® led to sustained improvements in pain, stiffness and physical function and mobility at Week 48 when compared with that at baseline in a double-blind placebo-controlled study.12
- In these phase 3 studies, CRYSVITA® had an acceptable safety profile up to 48 weeks in adults with XLH.12,14
Useful Websites
Publicly Available Websites
XLH information websites for healthcare professionals

1. XLHLink.asia (Healthcare professional website)
https://www.xlhlink.asia/hcp/
This website is developed and funded by Kyowa Kirin Asia Pacific Pte. Ltd.
XLH information websites for healthcare patients

1. XLHLink.asia (Patient website)
https://www.xlhlink.asia/
This website is developed and funded by Kyowa Kirin Asia Pacific Pte. Ltd.
Patient Organisations

1. Rare Disorder Society Singapore (RDSS)
RDSS was established in 2011 and aims to engage individuals and organizations, both private and public, to raise awareness on rare disorders and how we can all do a part to help patients living with rare disorders. The RDSS leadership team consists of members of the public with medical doctors acting as medical advisors.
https://www.rdss.org.sg
1. Beck-Nielsen SS, et al. Eur J Endocrinol. 2009;160:491–7. 2. Endo I, et al. Endocr J. 2015;62:811–6. 3. Carpenter TO, et al. J Bone Miner Res. 2011;26:1381–8. 4. Haffner D, et al. Nat Rev Nephrol. 2019;15:435–55. 5. Rafaelsen S, et al. Eur J Endocrinol. 2016;174:125–36. 6. Rajah J, et al. Eur J Pediatr. 2011;170:1089–96. 7. Raimann A, et al. Wien Med Wochenschr. 2020;170:116–23. 8. Razzaque MS. Nat Rev Endocrinol. 2009;5:611–9. 9. Linglart A, et al. Endocr Connect. 2014;3:R13–30. 10. Beck-Nielsen SS, et al. Orphanet J Rare Dis. 2019;14:58. 11. CRYSVITA® (burosumab). Based on Singapore Package Insert. Kyowa Kirin Asia Pacific Pte Ltd; May 2021. 12. Portale AA, et al. Calcif Tissue Int. 2019;105:271–84. 13. Imel EA, et al. Lancet. 2019;393:2416–27. 14. Insogna KL, et al. J Bone Miner Res. 2019;34:2183–91. 15. Carpenter TO, et al. N Eng J Med. 2018;378:1987–98. 16. Insogna KL, et al. J Bone Miner Res. 2018;33:1383–93