Start treatment with CRYSVITA® as early as possible after diagnosis of XLH in suitable patients
Table of Contents
As of 3 May 2021, CRYSVITA® has been approved by Health Science Authority (HSA) Singapore. To decide if this is a suitable option for any of your patients, please click here for more information.
Product is approved in EU/US/Japan and selected markets in Asia Pacific. Local approved prescribing information may differ. Please refer to local approval status and prescribing information before prescribing.
Dosing
CRYSVITA® dosing is every 2 weeks in pediatric patients (1 to less than 18 years of age)1
As of 3 May 2021, CRYSVITA has been approved by Health Science Authority (HSA) Singapore. To decide if this is a suitable option for any of your patients, please click here for more information.
Product is approved in EU/US/Japan and selected markets in Asia Pacific. Local approved prescribing information may differ.
Please refer to local approval status and prescribing information.
Dosing in pediatric patients (1 to less than 18 years of age) is every 2 weeks.
After initiating CRYSVITA®, measure fasting serum phosphate:
- every 4 weeks for the first 3 months of treatment, and
- thereafter as appropriate.
If serum phosphorus is above the lower limit of the reference range for age and below 5 mg/dL, continue treatment with the same dose. Follow dose adjustment schedule below to maintain serum phosphorus within the reference range for age.
If fasting serum phosphate is within the reference range for age, the same dose should be maintained.
Discontinue oral phosphate and active vitamin D analogs 1 week prior to initiation of treatment. Fasting serum phosphorus concentration should be below the reference range for age prior to initiation of treatment.
CRYSVITA® is dosed every 4 weeks in adult patients (18 years of age and older)1
After initiation of treatment with CRYSVITA®, fasting serum phosphorous should be measured:
- on a monthly basis, measured 2 weeks post-dose, for the first 3 months of treatment, and
- thereafter as appropriate.
If serum phosphorus is within the normal range, continue with the same dose.
Discontinue oral phosphate and active vitamin D analogs 1 week prior to initiation of treatment. Fasting serum phosphorus concentration should be below the reference range for age prior to initiation of treatment.
Calculation for starting dose
Sample Starting Dose Calculation – Pediatric (1 to <18 years old)
Patient weight (kg) x Recommended starting dose (0.8mg/kg)
Example: 23kg x 0.8mg/kg = 18.4 mg (Round to nearest 10 mg)
Starting dose CRYSVITA® = 20 mg
Sample Starting Dose Calculation – Adult patient (≥18 years of age)
Patient weight (kg) x Recommended starting dose (1mg/kg)
Example: 77kg x 1mg/kg = 77 mg (Round to nearest 10 mg)
Starting dose CRYSVITA® = 80 mg
Pediatrics Dose Adjustments1
Dose Increase (pediatric patients 1 to less than 18 years of age)1 :
If serum phosphorus is below the reference range for age, the dose may be increased stepwise up to approximately 2 mg/kg, administered every two weeks (maximum dose of 90 mg) according to the dosing schedule shown in Table 1.
Reassess fasting serum phosphate level 4 weeks after each dose adjustment.
Table 1. PEDIATRIC DOSE SCHEDULE
Pediatric Dose Schedule for Stepwise Dose Increase
| Body Weight (kg) | Starting Dose (mg) | First Dose Increase to (mg) | Second Dose Increase to (mg) |
|---|---|---|---|
| 10-14 | 10 | 15 | 20 |
| 15-18 | 10 | 20 | 30 |
| 19-31 | 20 | 30 | 40 |
| 32-43 | 30 | 40 | 60 |
| 44-56 | 40 | 60 | 80 |
| 57-68 | 50 | 70 | 90 |
| 69-80 | 60 | 90 | 90 |
| 81-93 | 70 | 90 | 90 |
| 94-105 | 80 | 90 | 90 |
|
| 90 | 90 | 90 |
Dose decrease (pediatric patients 1 to less than 18 years of age)1
Dose Decrease (pediatric patients 1 to less than 18 years of age)1 :
If serum phosphorus is above 5 mg/dL, withhold the next dose and reassess the serum phosphorus level in 4 weeks.
The patient must have serum phosphorus below the reference range for age to reinitiate CRYSVITA®. Once serum phosphorus is below the reference range for age, treatment may be restarted according to the dose schedule shown in Table 2.
Reassess serum phosphorus level 4 weeks after dose adjustment.
If the level remains below the reference range for age after the re-initiation dose, the dose can be adjusted according to Table 1.
Table 2. PEDIATRIC DOSE SCHEDULE
Pediatric Dose Schedule for Re-Initiation of Therapy
| Previous Dose (mg) | Re-initiation Dose (mg) |
|---|---|
| 10 | 5 |
| 15 | 10 |
| 20 | 10 |
| 30 | 10 |
| 40 | 20 |
| 50 | 20 |
| 60 | 30 |
| 70 | 30 |
| 80 | 40 |
| 90 | 40 |
Do not adjust CRYSVITA® more frequently than every 4 weeks
Adult Dose Adjustments1
Dose Decrease (adults 18 years of age and older)1 :
Reassess fasting serum phosphorus level 2 weeks after dose adjustment.
Do not adjust CRYSVITA® more frequently than every 4 weeks.
If serum phosphorus is above the normal range, withhold the next dose and reassess the serum phosphorus level after 4 weeks. The patient must have serum phosphorus below the normal range to be able to reinitiate CRYSVITA®. Once serum phosphorus is below the normal range, treatment may be restarted at approximately half the initial starting dose up to a maximum dose of 40 mg every 4 weeks according to the dose schedule shown in Table 3.
Reassess serum phosphorus 2 weeks after any change in dose.
Table 3. ADULT DOSE SCHEDULE FOR RE-INITIATION OF THERAPY
| Previous Dose (mg) | Re-initiation Dose (mg) |
|---|---|
| 40 | 20 |
| 50 | 20 |
| 60 | 30 |
| 70 | 30 |
|
| 40 |
Download the CRYSVITA® Dosing Guide for additional information on initiating, dosing, and administering CRYSVITA®.
Missed or late dosing1
If a patient misses a dose, resume CRYSVITA® as soon as possible at the prescribed dose.
Administration
CRYSVITA® Administration1
CRYSVITA® should be administered subcutaneously1
Discontinue oral phosphate and active vitamin D analogs 1 week prior to initiation of treatment. Fasting serum phosphorus concentration should be below the reference range for age prior to initiation of treatment
General Considerations for Subcutaneous Administration1
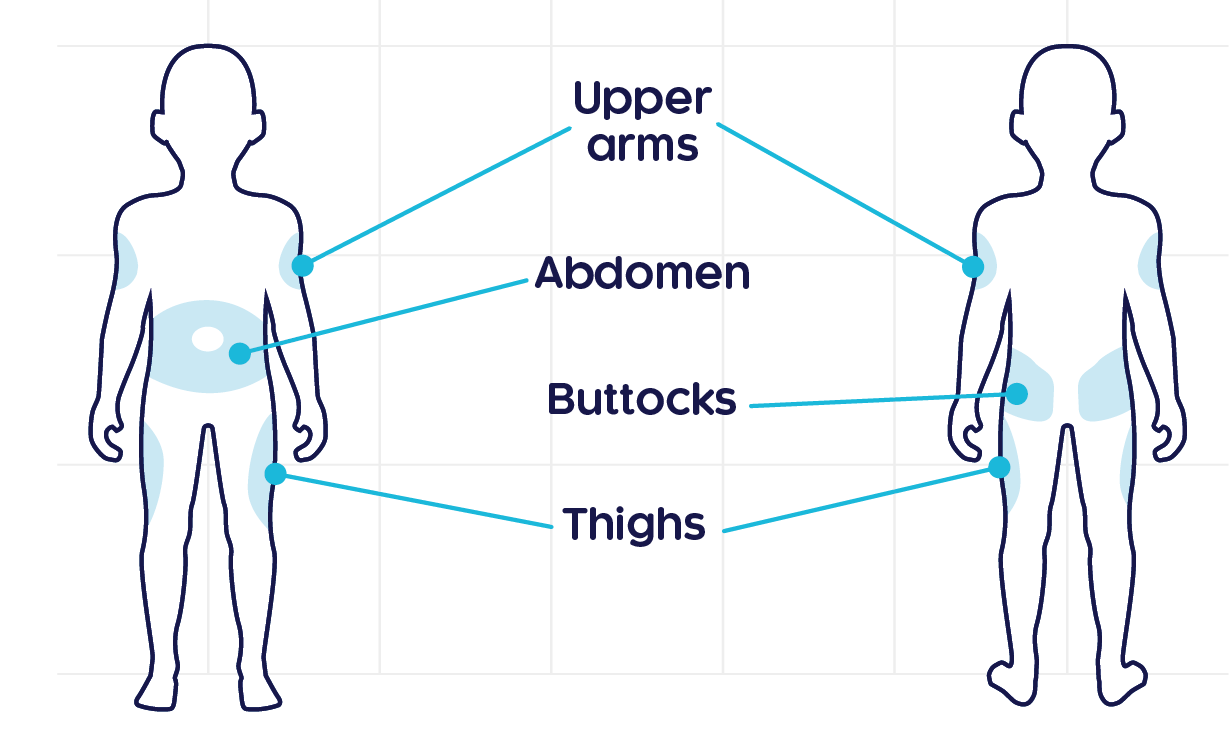
Injection sites should be rotated with each injection administered at a different anatomic location (upper arms, upper thighs, buttocks, or any quadrant of abdomen) than the previous injection. Do not inject into moles, scars, or areas where the skin is tender, bruised, red, hard, or not intact.
The maximum volume of CRYSVITA® per injection site is 1.5 mL. If more than 1.5mL is required on a given dosing day, the total volume of CRYSVITA® should be split and administered at two different injection sites. Monitor for signs of reactions.
Visually inspect CRYSVITA® for particulate matter and discoloration prior to administration. CRYSVITA® is a sterile, preservative-free, clear to slightly opalescent and colorless to pale brown-yellow solution for subcutaneous injection. Do not use if the solution is discolored or cloudy or if the solution contains any particles or foreign particulate matter.
Although you and your staff may be familiar with the preparation of drug suspensions and the administration of depot injections, it is important that you read all the instructions specifically administering CRYSVITA®. Clinical monitoring of the patient, including monitoring of phosphate levels, must continue as required and as outlined below. A detailed ‘Instructions for Use’ intended for Healthcare Professionals can be found, by clicking here.
Monitoring
Recommended monitoring schedule for CRYSVITA® patients2
| Assessment | Frequency |
|---|---|
| Fasting serum phosphate† | Month 1: Every 2 weeks |
| Month 2-3: Every 4 weeks | |
| Thereafter: As appropriate | |
| Following dose adjustment: 4 weeks after | |
| Renal ultrasonography | At least every 2 years in patients without nephrocalcinosis and at yearly intervals in patients with nephrocalcinosis and/or persistent hypercalciuria |
| Plasma ALP, calcium, PTH and creatinine | In children aged 1–5 years: Every 1-3 months, or as indicated |
| In children 5-12 years: Every 3-6 months, or as indicated | |
| Urine calcium and phosphate‡ | Every 3-6 months |
†It is recommended that fasting serum phosphate is targeted at the lower end of the normal reference for age.
‡Upper normal range (mol/mol): 2.2 (<1 years), 1.4 (1–3 years), 1.1 (3–5 years), 0.8 (5–7 years) and 0.7 (>7 years).
Contraindications, warnings and precautions1
Contraindications
- Do not use CRYSVITA® with oral phosphate and active vitamin D analogs.
- Do not initiate CRYSVITA® treatment if serum phosphorus is within or above the normal range for age.
- CRYSVITA® is contraindicated in patients with severe renal impairment or end stage renal disease because these conditions are associated with abnormal mineral metabolism.
Warnings
Hypersensitivity
Hypersensitivity reactions (e.g. rash, urticaria) have been reported in patients with CRYSVITA®. Discontinue CRYSVITA® if serious hypersensitivity reactions occur and initiate appropriate medical treatment.
Hyperphosphatemia and Risk of Nephrocalcinosis
Increases in serum phosphorus to above the upper limit of normal may be associated with an increased risk of nephrocalcinosis. For patients already taking CRYSVITA®, dose interruption and/or dose reduction may be required based on a patient’s serum phosphorus levels.
Injection Site Reactions
Administration of CRYSVITA® may result in local injection site reactions. Discontinue CRYSVITA® if severe injection site reactions occur and administer appropriate medical treatment.
Special Populations
Pregnancy
There are no available data on CRYSVITA® use in pregnant women to inform a drug-associated risk of adverse developmental outcomes. Serum phosphorus levels should be monitored throughout pregnancy.
The background risk of major birth defects and miscarriage for the indicated population is unknown; however, the estimated background risk in the U.S. general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Lactation
There is no information regarding the presence of burosumab in human milk, or the effects of CRYSVITA® on milk production or the breastfed infant. Maternal IgG is present in breast milk. However, the effects of local gastrointestinal exposure and limited systemic exposure to CRYSVITA® in the breastfed infant are unknown. The lack of clinical data during lactation precludes a clear determination of the risk of CRYSVITA® to an infant during lactation. Therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CRYSVITA® and any potential adverse effects on the breastfed infant from CRYSVITA® or from the underlying maternal condition.
Pediatric Use
Safety and efficacy of CRYSVITA® have been established in pediatric patients 1 year and older. Safety and effectiveness in pediatric patients 1 year and older with XLH are based on one phase 3, open-label, active control study [61 patients 1-12 years of age (Study 1)] and two open-label studies [52 patients 5 to 12 years of age (Study 2), and 13 patients 1 to 4 years of age (Study 3)] evaluating serum phosphorus and radiographic findings. Safety and effectiveness in adolescents are supported by evidence from the studies in pediatric patients 1 year to less than 13 years of age with additional modeling and simulation of adult and pediatric pharmacokinetic and pharmacodynamic data to inform dosing. Safety and efficacy for CRYSVITA® in pediatric patients with XLH below the age of 1 have not been established.
Geriatric Use
Clinical studies of CRYSVITA® did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Storage
CRYSVITA® Storage1
Store CRYSVITA® away from light and in refrigerated conditions1
- CRYSVITA® is supplied as a sterile, preservative-free, clear to slightly opalescent and colorless to pale brown-yellow solution for subcutaneous injection. The product is available as one single-dose vial per carton in the following strengths: 10, 20, and 30 mg/mL.*
- Visually inspect CRYSVITA® for particulate matter and discoloration prior to administration.
- Do not use if the solution is discolored or cloudy or if the solution contains any particles or foreign particulate matter.
*Not all presentations may be available locally
CRYSVITA® vials must be stored in the original carton until the time of use under refrigerated conditions at (2°C to 8°C). Keep CRYSVITA® vial in the original carton to protect from light until time of use.
Do not freeze or shake. Do not use CRYSVITA® beyond the expiration date stamped on the carton. CRYSVITA® vials are single-dose only. Discard any unused product.
Please refer to the full Prescribing Information for full details before prescribing.
1. CRYSVITA® (burosumab). Based on Singapore Package Insert. Kyowa Kirin Asia Pacific Pte Ltd; May 2021 2. Haffner D et al. Nat Rev Nephrol. 2019; 15(7): 435–455.






